If the behaviour of a copper steel and a stainless steel steel couple is compared the copper steel coupling is a more significant galvanic problem despite the similar potential separation of 0 35 volts.
Galvanized and stainless steel reaction.
The presence of two dissimilar metals in an assembly is not always a sign of trouble but it could be a problem.
The efficiency of this cathodic reaction will determine the corrosion rate.
Under atmospheric conditions of moderate to mild humidity contact between a galvanized surface and aluminum or stainless steel is unlikely to cause substantial incremental corrosion.
Chromium can shrug off oxygen without corroding.
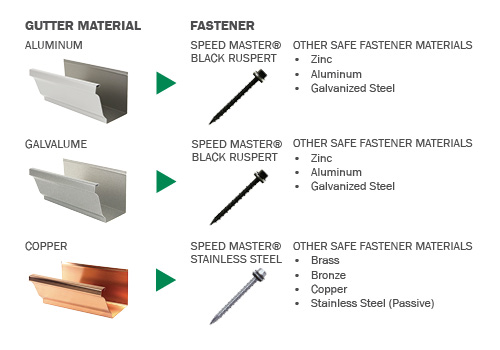
Since brass doesn t react with either the copper or the zinc one method is to make sure you have a threaded female end on.
Stainless steel has a secret weapon.
Stainless steel has an effective passive film so the available corrosion current able to be carried by charged atoms ions is quite low.
However under very humid conditions the galvanized surface may require electrical isolation from the aluminum or stainless steel.
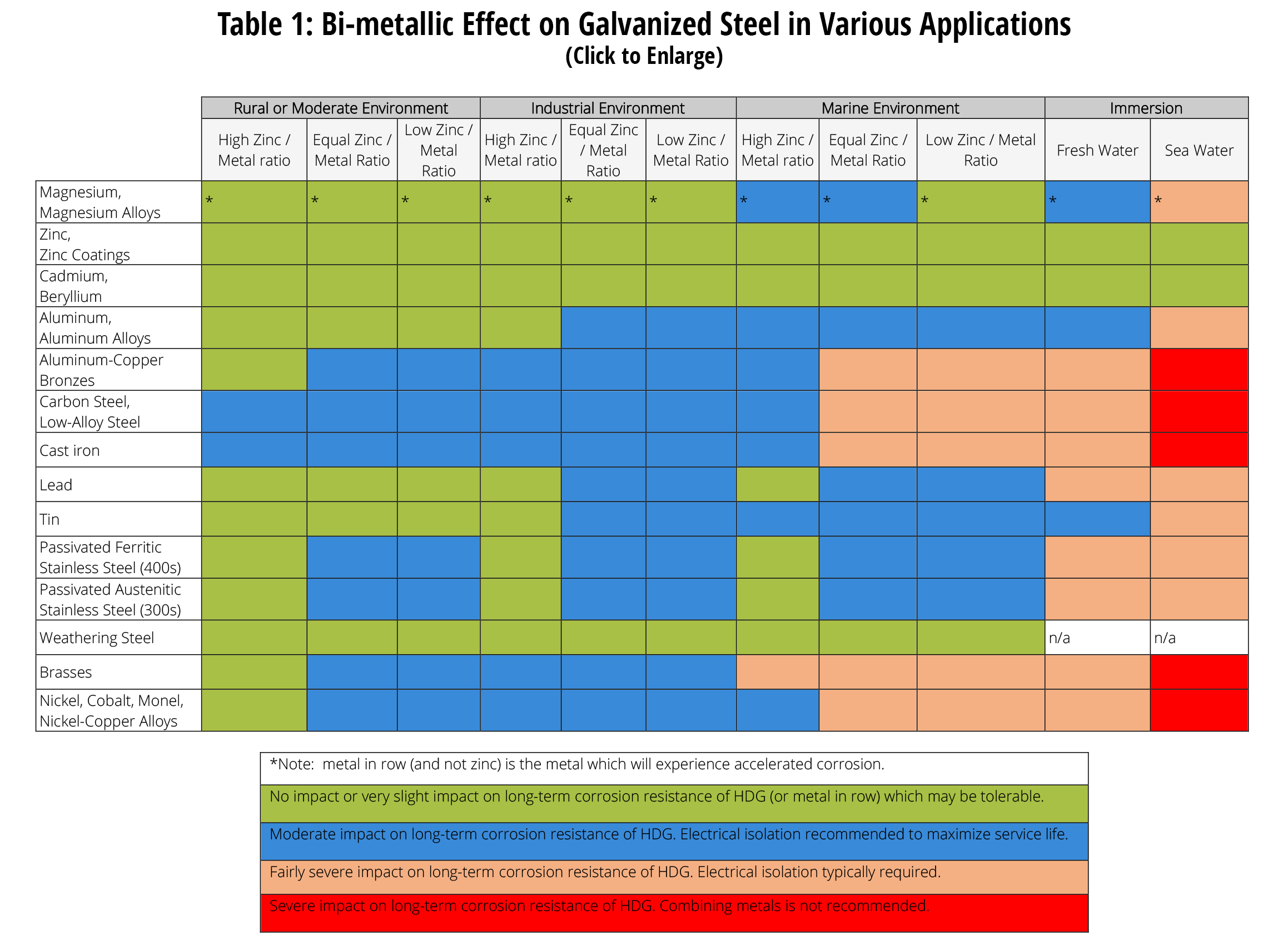
Steel zinc plated stainless steel type 410 stainless steel type 302 304 316 aluminum base metal zinc galvanized zn al coated steel a c c b aluminum a 1not recommended b a steel cast iron a d c b a brass copper bronze a d e a b a e stainless steel 300 series a d e a a a e footnotes 1.
Aluminum and stainless steel.
Galvanic action between galvanized and stainless steel.
The addition also pushes stainless steel higher up on the nobility chart.
Please understand that green represents lower risk not no risk it should be noted that if sacrificial plating is incorporated in the fastener design then galvanic action can result in the deterioration of the sacrificial coating rather than of the fastener.
Carbon steel can.
A discussion started in 2001 but continuing through 2019.
The most common examples of galvanic corrosion of aluminum alloys are when they are joined to steel or copper and exposed to a wet saline environment.
To safely connect copper and galvanized steel you have two options.
The galvanic corrosion is very dependent on the cathode reaction and which metals are in contact with each other.
So when stainless steel and carbon steel are connected and an electrolyte such as moisture is introduced stainless steel absorbs carbon steel s electrons.